
See how the ReVENT® Sleep
Apnea System works.
The procedure is performed by an ENT surgeon and has a quick recovery time.
The ReVENT Sleep Apnea System is a minimally invasive implant for the treatment of Obstructive Sleep Apnea (OSA). The procedure is performed by an ENT surgeon and has a quick recovery time. It addresses the non-compliant issues associated with CPAP therapy. Additionally, the procedure is reversible if required.
 The system is intended for use in stabilizing the tongue for the reduction of the incidence of tongue based airway obstruction in patients suffering from OSA.
The system is intended for use in stabilizing the tongue for the reduction of the incidence of tongue based airway obstruction in patients suffering from OSA.
The system addresses tongue based obstruction while addressing the non-compliance issues often associated with Continuous Positive Airway Pressure (CPAP) and other daily therapies. The implants are inserted using a minimally invasive technique and are designed to provide a light spring-like force to the tissue. After the implants heal into place with the looped ends acting as an anchoring mechanism, the bio-absorbable sections between the looped ends of the implants erode allowing the implants to gently contract over time. The spring-like force is designed to maintain an open airway, but not be noticed by the patient.
The ReVENT Sleep Apnea System consists of implants and an implanter kit. The implant is intended for permanent implantation in the tongue base and dynamically supports the tissue after healing. The procedure is performed by an ENT surgeon and has a quick recovery time.
ReVENT Sleep Apnea System Components
- Tongue Implanter Kit
- Implants
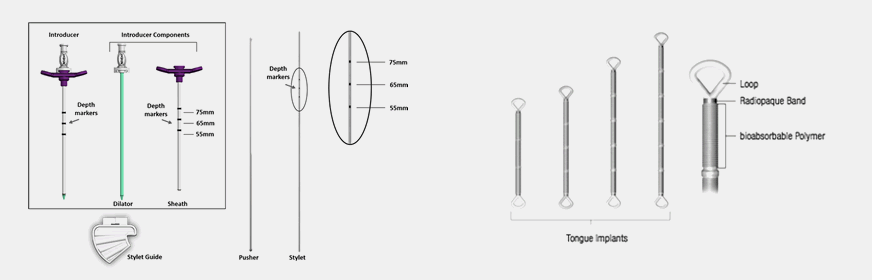
The implant is a silicone elastomer with loops at each end. The loops are designed to allow tissue healing to anchor the implant in place. The loops also include radiopaque markers for locating the implants post-implementation, if required. The implant body contains segment of bio-absorbable polymer which are absorbed by the body during healing, initiating the dynamic system.
The ReVENT Sleep Apnea System has received CE Mark and is available on a limited basis in Europe. It is also currently being clinically evaluated in Canada.

